Chemical Energy → Electric Energy
To generate energy, the source components participating in the process should have more energy than the final components of the process. In case of generation of any form of energy from chemical energy we deal with chemical reaction, during which the inter-atomic energy of source components should exceed the inter-atomic energy of products of chemical reaction.
The simple form of such process is burning that produces heat. For example, burning methane CH4 in atmosphere that contains oxygen O2 is a chemical reaction described by
CH4 + 2O2 = CO2 + 2H2O
The inter-atomic energy of one molecule of methane and two molecules of oxygen must be greater than inter-atomic energy of a molecule of carbon dioxide and two molecules of water, otherwise there will not be any energy released as heat.
To generate electric energy from chemical we need the same type of inequality: the inter-atomic energy of primary components of the chemical reaction must be greater than inter-atomic energy of the resulting components.
The way how this excess of energy represented depends on the chemical reaction. In the process of burning the excess of energy is in a form of heat, in case of the generating of electricity the excess of energy is in a form of electric current that has certain voltage and amperage and, therefore, is a carrier of energy.
Transformation of chemical energy into electricity is typically occurring in batteries.
There are different types of batteries, and in this lecture we will mention a few with some details of how they work.
Consider a lead-acid battery used in many cars.
In a simplified way it has three major components: solid anode (negative electrode) made of lead Pb, solid cathode (positive electrode) made of lead dioxide PbO2 and liquid electrolyte in-between them containing sulfuric acid H2SO4 diluted in water H2O.
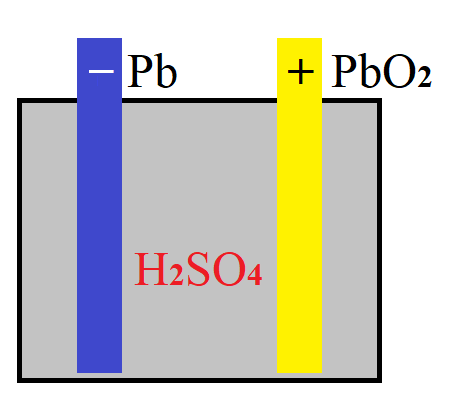
To understand why electricity is generated by this device, let's look inside the atomic structure of its components and analyze what happens when there is a load (like a lamp) connected to its terminals.
Recall the classical planetary model of an atom.
There are protons and neutrons forming its nucleus and electrons circulating around this nucleus on different orbits.
Those electrons on outer most orbits are less attached to a host nucleus and many of them can fly around, potentially getting attached by other host nuclei.
This creates certain amount of "free" positively (with deficit of electrons) charged ions called cations and negatively (with access of electrons) charged ions called anions.
Analogously, molecules are also not completely stable and can lose atoms, if inter-atomic forces are not strong enough.
Applied to molecular structure of any substance, typical contents not only contains electrically neutral molecules, but also molecules with certain missing or extra atoms or electrons - ions.
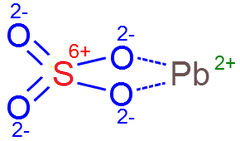
Consider sulfuric acid inside a battery pictured above.
Structural composition of atoms of each molecule of this acid can be represented as

Many of electrically neutral molecules of sulfuric acid H2SO4 are partially breaking losing a positive ions (nuclei with a single proton) of hydrogen but retaining their electrons, which can be represented as
H2SO4 → 2H + + SO42−
This can be explained by the fact that hydrogen nucleus contains only one proton, this is the lightest and electrically weakest nucleus and, being so light and volatile, it easily breaks its inter-atomic links with the main molecule of sulfuric acid. The resulting cations H + and anions SO42− are actually responsible for burning and corrosion caused by sulfuric acid. In batteries, however, these ions are responsible for producing electricity.
Here is how.
The negative anode of a lead-acid battery is made of lead (chemical symbol Pb), which, when going into chemical reactions, exhibits either 2 or 4 links to other atoms in the molecules.
The negative ion (anion) SO42−, produced during the breaking of the molecules of sulfuric acid, chemically reacts with led producing lead sulfate, and releasing two electrons left after ions of hydrogen broke free from the molecule of sulfuric acid as follows:
Pb+SO42− → PbSO4 + 2e−
Structural composition of atoms of each molecule of lead sulfate can be represented as
Keep an eye on two electrons 2e− produced at anode. They are produced on the surface of a lead anode and accumulated inside it up to a certain concentration that gives certain negative charge to an anode. These electrons will be the ones that produce the electric current, when some load (like a lamp) is connected to terminals of a battery.
Meanwhile, at the cathode terminal of a led-acid battery another reaction goes on between its main component lead dioxide PbO2, having the following structure
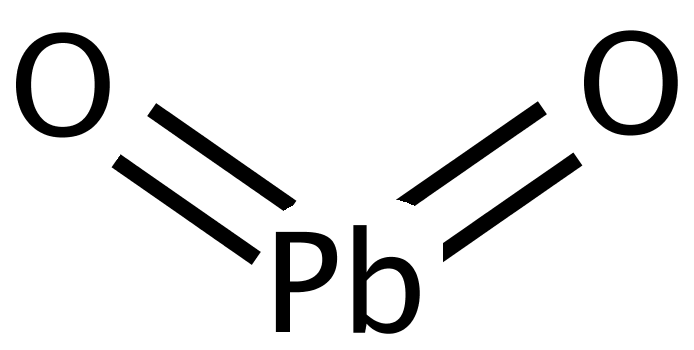
and electrolyte that still contains unused positive ions of hydrogen 2H+ from the reaction on an anode and another pair of ions, positive 2H+ and negative SO42− of the sulfuric acid.
Now two reactions simultaneously go on at the surface of a cathode.
First of all, lead dioxide connects with negative ion of sulfuric acid, producing lead sulfate and releasing two negative ions of oxygen:
PbO2 + SO42− →
→ PbSO4 + 2O−
Secondly, remaining two positive ions of hydrogen from a molecule of sulfuric acid participating in the above reaction and two positive ions of hydrogen from the reaction on an anode meet two negative ions of oxygen from the above reaction, forming two molecules of water H2O with two electrons still missing:
4H+ + 2O− → 2H2O2+
Concentration of these molecules of water missing two electrons creates a positive charge on the cathode terminal of a battery. When this concentration reaches certain level, positive ions of hydrogen cannot reach a cathode, ions of hydrogen are not readily breaking off the molecules of sulfuric acid and reaction stops at certain level of positive charge, unless we connect anode and cathode through some external electric load to allow accumulated on an anode electrons compensate missing electrons on a cathode.
So, we have a shortage of two electrons on a cathode terminal of a battery to form electrically neutral molecules of lead dioxide and water, but these two electrons can travel through an outside load between the terminals of a battery from its anode.
The overall reaction on a cathode looks like
PbO2 + 4H+ + SO42− + 2e− →
→ PbSO4 + 2H2O
As a result of these chemical reactions electrons released by a reaction on an anode travel to a cathode through external load, thus creating an electric current. In an absence of an external load reactions on terminals of a battery continue until some limit of concentration of negative charge on an anode and positive charge on a cathode is reached, after which the reactions stops as further ionization is prevented by accumulated charges.
This is how chemical energy (inter-molecular links) is converted into electrical energy in a lead-acid battery.
The above described lead-acid battery is capable to work in reverse, to accumulate chemical energy, if external electromotive force is applied to its terminals. In this case all reactions go in reverse, that's how car battery is charged by alternator, when a car is in motion.
The overall picture of the lead-acid battery to discharge and charge is as follows
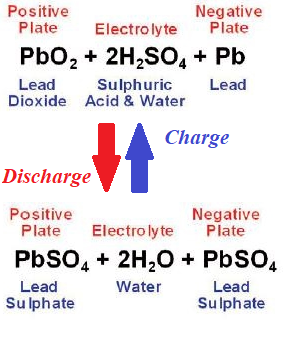


No comments:
Post a Comment