Laser
We all are familiar with laser scanners used in stores to scan the product code.
Many of us know about laser pointers that direct a small spot of light on objects quite far away.
Compact discs technology is based on lasers.
Laser printers are nowadays the dominant printing device used in computers.
There are many other applications of lasers, so, let's examine the laser's principles of working.
First of all the term laser is an abbreviation of "Light Amplification by the Stimulated Emission of Radiation". This name fully characterizes the process at the base laser's functionality, and here is why.
Atom Model
We will use a familiar model of an atom with a nucleus in a center and electrons circulating around it on different distinct orbits or, to better describe their place in three-dimensional space, in different distinct shells, with each shell containing certain number of electrons up to some maximum, specific for each shell of each element.
All electrons within any particular shell have the same level of energy.
The composition of shells is modeled as concentric spheres around a nucleus, each characterized by a specific energy level of electrons in it.
Contemporary view based on Quantum Theory, states that radii of these spheres can take only discrete values specific for each element.
When an electron absorbs energy from some outside source, it jumps from a lower energy level shell to a higher energy level one.
When it moves in an opposite direction, jumping back to a shell of a lower energy level, it emits radiation.
Atom of each element can absorb or emit energy in small but finite chunks, quanta, the size of which depends on the element's characteristics, in particular, on the discrete energy level differences between its different shells.
Absorption of a single quantum of energy by an atom manifests itself in one of its electrons jumping to a higher energy level shell with the shell energy level increment equal to the absorbed quantum of energy.
Emitting a quantum of energy is related to an electron changing its position from a higher energy level shell to a lower energy level one, with the decrease in energy level corresponding to an amount of energy emitted.
Atom emits energy as electromagnetic oscillations of certain frequency. In some cases this frequency for some elements corresponds to a frequency of a visible light of some color, in other case it might fall in the ultraviolet, infrared or other part of a spectrum.
If an electron of some element jumps from a shell with a higher energy level Ehigh to a shell with a lower energy level Elow, it emits the extra energy as a quantum of electromagnetic oscillations called photon of a specific frequency f, related to a difference in the levels of energy as
Ehigh − Elow = h·f
where energy is measured in Joules (J),
h = 6.62607015·10−34(J·s) is the Planck's constant and
f is a frequency in hertz (Hz).
The Idea
Generally speaking, all electrons of an atom of any element in a stable state occupy certain shells called ground level shells.
The atom of hydrogen with 1 electron has one ground energy level shell. The atom of sodium has 11 electrons positioned in 3 ground energy level shells: (2+8+1).
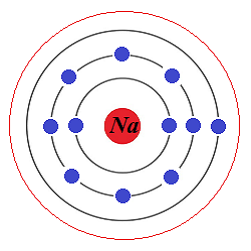
Let's assume that we supply some constant external energy to atoms of some element that causes some of its electrons on the outer ground energy level shells to get excited and move to a higher energy level shells.
By supplying energy in quanta that exactly equal to a difference in the levels of energy between a ground energy level shell (a stable state of electrons), characterized by the energy level Eground, and a higher energy shell (excited state of electrons), characterized by the energy level Ehigh, we force many electrons to absorb this energy, get excited and move from the ground level shell to the higher energy level shell (energy must be conserved!), eventually overpopulating that higher energy level (not that stable) shell.
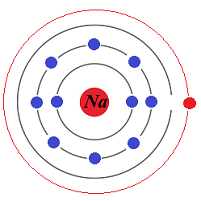
The trick is to force these electrons, that overpopulate the higher energy level shell, to synchronously relax and jump back to a ground level shell, returning to a stable state.
If this is achieved, they synchronously emit a photon of radiation of the same frequency and phase producing a coherent (synchronized in frequency and phase) light.
Theoretical Solution
Assume, we have an overpopulated higher energy shell of energy level Ehigh.
Experiments showed that, when a single photon of frequency f carrying an energy
E = h·f = Ehigh−Eground
falls onto one of the excited electrons in the overpopulated higher energy shell,
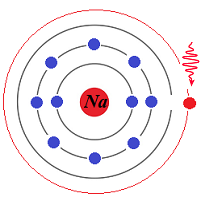
it is not absorbed by an already excited electron, but stimulates this electron to relax by going down to a ground level shell of the energy level Eground, emitting a photon of corresponding frequency
f = (Ehigh−Eground)/h
(energy must be conserved!)
which is the same as the frequency of the incident photon and is also synchronized with it in phase.
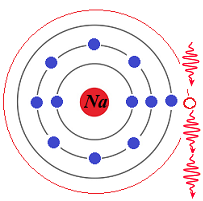
The above is a description of Stimulated Emission of Radiation, the second part of the full name that produced an abbreviation laser.
Now we have two identical in frequency and phase photons, the incident one and the emitted by an electron that switched its position from the high energy level shell to the ground shell.
If we can redirect these two identical photons back to electrons on high energy level shell, they will cause two new electrons to relax, emitting two more photons fully synchronized with incident ones, thus enhancing the light, while maintaining the same frequency and phase for all photons.
We can repeat this redirection of new photons back to excited electrons, and on each repetition the number of emitted photons would, in theory, double.
Eventually, when this light is strong enough, we can release it and use for some purpose.
If there is an external source of energy that constantly excites electrons, which, in turn, forces them to jump to a high energy level shell and, at the same time, we can stimulate these electrons to relax, emitting extra energy in a form of electromagnetic waves of certain frequency and phase (the same for all relaxing electrons in all atoms of an element), we will obtain a flow of coherent (all of the same frequency and phase) electromagnetic oscillations.
If the frequency of these oscillations corresponds to a visible spectrum, we will get a coherent light of some color.
Practical Aspects
Exact details of implementation of the above idea are beyond the scope of this course, but a few possible technical details of the implementation can be suggested.
Imagine a tube with some gas and two electrodes in it connected to a battery. One of the most commonly used gas laser uses a mixture of helium (He) and neon (Ne) gases. The battery should be sufficiently powerful to establish a flow of electric current through this gas.
If the voltage of a battery is sufficient, this construction allows the gas atoms to constantly absorb the energy and, after a while, the gas might heat up (the molecules will increase their chaotic movement within a tube) and might emit some visible light to compensate for overloaded with energy electrons, that, after being excited to a limit, relax and release the extra energy as randomly emitted photons.
Let's make one end of this tube fully reflective and the opposite - partially reflective, that is, it's reflective for low level energy light, but the high energy ray of light will break through and go out through this end of a tube.
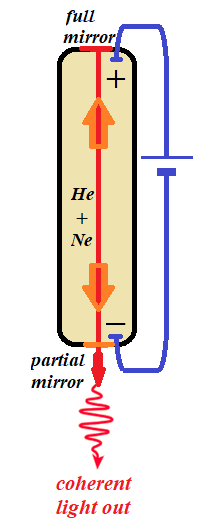
Now, if we inject into this device a single photon of proper frequency level that corresponds to a difference in energy levels of electrons between excited and stable condition or just wait until some electron will spontaneously jumps down, emitting a needed photon, this photon will cause one of the excited electrons to relax, jump to a ground level shell and emit the photon identical to an incident one. That's what stimulated emission means.
The two photons, original and emitted, will not have enough energy to go through a partially reflective end of a tube and will just hit two previously excited electrons, causing emission of two new photons of the same frequency and phase. We have four photons now.
Continuing this "chain reaction" of producing more and more photons, the total energy of light grows until it's strong enough to go through a half-reflective end of a tube as the bright coherent light.
Actually, there are many different ways to implement the laser. Gas tube is not the only one. There are lasers built with liquid substance, solid substances, semiconductors etc.
The energy source might not only be an electric battery, but some bright light as well.
One of the applications of laser technology is military weapon system to destroy drones and other flying objects with high precision and minimum expenses.


No comments:
Post a Comment