α, β, γ Decay
Experiments with radioactive elements (primarily, radium) around 1900 performed by Rutherford and Villar resulted in detection of some kind of rays emitted by a radioactive element.
These rays were subdivided into three kinds - those that carry positive electric charge (α-rays), those negatively charged (β-rays) and electrically neutral (γ-rays).
It was concluded that radioactivity is some kind of atom's transformations occurred under certain circumstances.
In this lecture we will address three types of transformations called α-, β- and γ-decay.
These transformations may occur spontaneously or as a result of some external force, like bombarding a nucleus with neutrons or under extreme heating etc.
α-Decay
An α-particle is a combination of two protons and two neutrons, which is the same as a nucleus of an atom of helium 2He4.
An α-decay is a process of spontaneous or forced emitting an α-particle from a nucleus of an atom, thus converting this nucleus into a different type.
Usually, an α-decay is observed with heavy elements. For example, an isotope of uranium-238 can spontaneously undergo an α-decay converting into an isotope of thorium-234:
92U238 → 90Th234 + 2He4
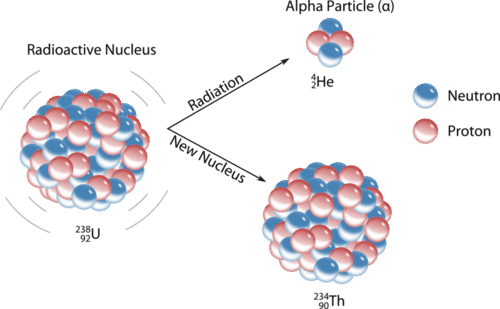
An emitted α-particle is relatively heavy and does not travel far. It cannot penetrate a sheet of paper or human skin. However, if inside a body, α-particles can do significant tissue damage.
The famous case of poisoning of Russian spy Alexander Litvinenko with radioactive polonium is an example of such case.
Unstable radioactive isotope of polonium 84Po210 undergoes a spontaneous α-decay into a stable isotope of lead 82Pb206:
84Po210 → 82Pb206 + 2He4
emitting α-particles.
When α-decay occurs spontaneously, there are certain statistics involved. Not every atom undergoes a decay, but every atom has certain probability of decaying during a certain period of time. To quantify this process, physicists use a concept of half-life - the average amount of time half of the atoms will decay.
For example, half-life of polonium 84Po210 is relatively short period of 138 days, so in the case of Litvinenko poisoning, in a couple of weeks he had a pretty damaging amount of α-particles destroying his cells, and he died.
β-Decay
Some experiments with radioactive materials resulted in detection of particles called β-particles at the time.
Later on it was determined that these particles are electrons.
At the same time, emitting electrons should create an imbalance of electrical charge of the atom, since the negative charge goes away.
The situation with β-decay is, therefore, more complex than seems on a surface.
The further experiments showed that the atomic mass of the radioactive element remained the same during β-decay, while its electric state remained neutral.
The only possible reason for this was a transformation of one of the neutrons in a nucleus into a proton and an electron emitted from the atom.
In a simplified form one of the examples of β-decay (symbolized as β−) that involves unstable isotope of carbon-14 transforming into a stable isotope of nitrogen-14 is as follows:
β−: 6C14 → 7N14 + e− + ?
where ? at the end means that there are other particles in this reaction (in this case, anti-neutrino) not related to electric charge or atomic mass.
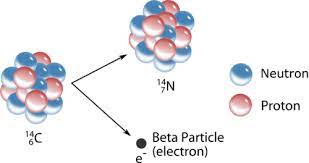
Emitting one electron with simultaneous transformation of a neutron into a proton maintains the atomic mass and atom's electric neutrality.
γ-Decay
The γ-decay is a different kind of a process. Historically, it was called "gamma" as one of the three kinds of detected rays emitted by radioactive material:
alpha for positively charged (later on identified as a combination of four particles - two protons and two neutrons),
beta for negatively charged (identified as electrons) and
gamma for electrically neutral rays.
These electrically neutral γ-rays were later on identified as very high frequency oscillations of electromagnetic field that behaved like particles because of duality of the electromagnetic field, which, especially in very high frequencies, can be viewed as a flow of particles - photons.
While the penetrating abilities of α and β-particles are very limited (a thin sheet of plastic or aluminum foil can stop them) those of γ-particles are extremely high. The γ-radiation can penetrate walls and, if it's of high intensity, is dangerous for living organisms.
The real protection from penetrating γ-radiation is a layer of lead.
The γ-radiation is not related to material particles, like protons or neutrons in case of α-particles or electrons in case of β-particles.
The γ-particles are photons that have no mass and are just lumps of energy.
So, to emit this energy, a nucleus should be in some kind of excited state and, emitting the γ-rays, the nucleus transforms into a stable state.
In simple terms this process of emitting γ-radiation can be explained as follows.
When α- or β-decay occurs, the balance of mass and energy between original atom and all the components of the decay changes. The total original mass and energy of the atom is now distributed among the resulting atom and the products of the decay.
Mass defect will now play a significant role, but some extra energy might still be left in the nucleus of the original atom.
This extra energy puts a nucleus in an excited state that causes emitting γ-radiation as pure lumps of energy (high frequency photons), thus transforming an atom into a stable state.
Because of the high penetrating properties of γ-radiation it can be used for some purposes. For example, in radiotherapy to treat cancer, to detect oil and gas deep under the surface of the Earth, even to change the inner structure of materials by exposing them to γ-radiation.


No comments:
Post a Comment