Fusion Basics
As we know, splitting (fission) of a nucleus of uranium-235 or plutonium-239 produces nuclei of combined mass smaller than initial mass of a nucleus of uranium-235 or plutonium-239.
It's called mass defect and it's the source of energy that can be used for different purposes, including, if not controlled, for making an atomic bomb.
Mass defect can be not only a result of a fission of nuclei of heavy elements, like uranium-235, but also from a fusion of light elements, as presented on this picture
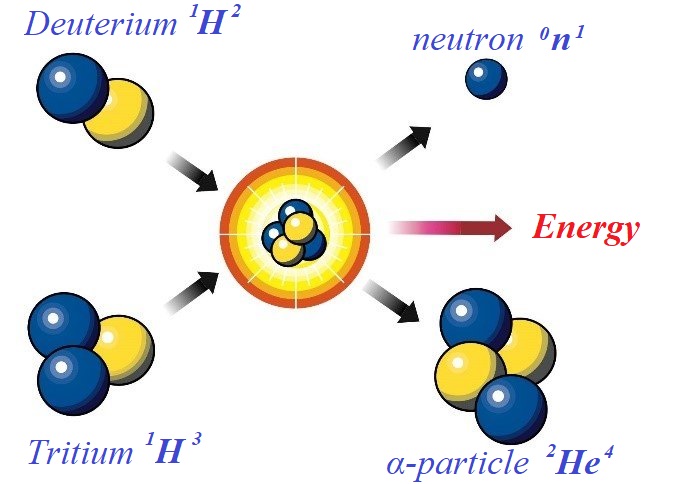
Here an isotope of hydrogen with one proton and one neutron in a nucleus 1H2 called deuterium is fused with another isotope of hydrogen with one proton and two neutron in a nucleus 1H3 called tritium.
The result of this fusion is an α-particle (that is, a nucleus of helium-4) 2He4 and free neutron 0n1 which can be expressed as an equation
1H2 + 1H3 = 2He4 + 0n1
Let's evaluate a mass defect of this process of fusion.
Mass (in atomic mass units) of a nucleus 1H2 is 2.014 amu.
Mass of a nucleus 1H3 is 3.016 amu.
So, total mass of the initial components of a fusion is 5.030 amu.
Mass of a nucleus 2He4 is 4.0015 amu.
Mass of a neutron 0n1 is 1.00867 amu.
So, total mass of the products of a fusion is 5.010 amu.
As we see, the products of fusion have smaller mass (that is, smaller amount of matter) than the initial components. The mass defect is Δm=0.02 amu, which is converted into energy according to Einstein's formula E=Δm·c².
Problems in Fusion
The first problem is where to get the fusion components deuterium 1H2 and tritium 1H3.
While deuterium is a stable isotope of hydrogen and can be extracted from water, tritium does not occur naturally and must be manufactured, which takes some energy.
The second problem is the physical conditions required for a reaction of fusion.
This reaction requires extremely high temperature (millions of degrees, like 100 million of °K) and pressure to squeeze the atoms to be within a very short distance from each other. To provide these conditions requires a lot of energy.
In attempts to create a controlled fusion physicists had to spend substantial amount of energy to achieve a successful fusion. Until recently, the net energy produced by controlled fusion was negative. However, some late experiments showed a small positive energy production. So, there is a hope of success in producing energy by controlled fusion.
On the other hand, uncontrolled fusion was achieved long time ago and implemented as an H-bomb. The needed temperature and pressure were provided by uncontrolled fission of uranium-235 or plutonium-239, described in the previous lectures.
Our Sun has the physical conditions required for a fusion to take place and plenty of necessary material for it - hydrogen. The energy emitted by Sun is the product of uncontrolled fusion.
Under these conditions different fusion reactions occur, not only deuterium to tritium. One of them involves hydrogen fused into helium through sequence of transformations, releasing energy as electromagnetic oscillations of different frequencies from γ-rays to infrared radiation:
(a) two nuclei of hydrogen are fused into a nucleus of deuterium emitting a positron (a positively charged anti-electron) and electrically neutral massless neutrino, releasing certain amount of energy on the way
1H1 + 1H1 → 1H2 + β+ + ν
where one of the protons of a hydrogen nuclei is transformed into a neutron and a positron with a neutrino as a by-product.
(b) the next reaction can occur between a freshly produced deuterium and hydrogen producing an helium-3 isotope, releasing certain amount of energy on the way
1H1 + 1H2 → 2He3
(c) two nuclei of a helium-3 isotope produced above fuse together forming a stable nuclei of helium-4 and hydrogen, releasing certain amount of energy on the way
2He3 + 2He3 → 2He4 + 2·1H1
At the end of this cycle, if we count all the participating nuclei, we see that from six nuclei of hydrogen a new nucleus of helium and two new nuclei of hydrogen are produced with certain amount of energy released on each step and different elementary particles emitted.
In a rather simplified form our Sun functions by burning hydrogen, producing helium and releasing energy on the way.
Provided temperature and pressure of Sun, many other nuclear reactions happened there, though they play a lesser role in producing energy.


No comments:
Post a Comment